Thus a reaction mixture containing a solvent with a high value for tan δ at standard operating conditions is recommended for an efficient. Xi-Sheng Wang Journal of the American Chemical Society 2022 144 14 6543-6550 Article Publication Date Web.

Ethylene Glycol Properties Uses Structure Britannica
A solution of 60 ethylene glycol in water.

. Ethanol serves this process by acting as a competitive inhibitor against methanol and ethylene glycol for alcohol. Higher values for tan δ represent a higher capacity to absorb microwaves. The two OH groups lead to extensive intermolecular hydrogen bonding.
It is used as a solvent a coolant in automotive radiators a deicing fluid for aircraft and a building block in the synthesis of polyester fibers and resins The current global ethylene glycol production capacity is approximately 42 million metric tons per year and is forecast to. Here the authors develop a tunable ternary. It is also completely miscible with water.
Ethylene glycol is a commodity chemical with applications in many areas of everyday life. Chain initiationusually by means of an initiator which starts the polymerization process. N1 ROH 2 n RCOOH 2 HOROOCRCOO n ROH 2n H 2 O.
In addition polymerization sometimes called chain-growth polymerization a chain reaction adds new monomer units to the growing polymer molecule one at a time through double or triple bonds in the monomer. Appl Microbiol Biotechnol 98. The medicinal properties of ethanol were studied by Arnald of Villanova 12401311 CE and John.
Deep eutectic solvents DES are intriguing green reaction media for biomass processing however undesired lignin condensation is a typical drawback. Quantitative and qualitative studies have identified a wide variety of chemical components in the cartridges refill solutions and aerosols of e-cigarettes. Synthesis includes adding together monomers in a long chain.
One monomer connects to the next and so on when a catalyst is introduced in a process known as chain growth polymers adding one monomer unit at a time. The polymerization process takes place in three distinct steps. Ethylene glycol is the main ingredient in many antifreeze mixtures for automobile radiators.
C Roth et al Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Polym Degrad Stabil 121 100104 2015. In almost all uses the synthesis method of the nanomaterials represents one.
Thus ethylene glycol does not boil away when it is used as an antifreeze. H Wu et al Synthesis and degradability of copolyesters of 2 5-furandicarboxylic acid lactic acid and ethylene glycol. Herrington and Myers 2015 have detected.
Polyesters can be obtained by a wide range of reactions of which the most important are the reaction of acids and alcohols alcoholysis and or acidolysis. Synthesis of polyesters is generally achieved by a polycondensation reaction. Enantioselective Synthesis of Secondary β-Trifluoromethyl Alcohols via Catalytic Asymmetric Reductive Trifluoroalkylation and Diastereoselective Reduction.
Describe a method for concentrating ethanol involving repeated fractional distillation through a water-cooled still by which an ethanol purity of 90 could be obtained. In general e-cigarettes often contain ingredients such as propylene glycol PG and glycerol mixed with concentrated flavors and optionally a variable percentage of nicotine. These authors describe a two-step size sorting process in order to obtain significant amounts of nanometric monosized particles with diameters between typically 6 and 13 nm.
FREE shipping and the BEST customer service. As the surface of the latter is not modified by the size sorting process usual procedures are used to disperse. Bing-Bing Wu Jie Xu Kang-Jie Bian Qian Gao and.
The general equation for the reaction of a diol with a diacid is. This results in a high boiling point198C. FREE shipping and the BEST customer service.
Some addition polymerisation reactions are considered to create no side-products and the reaction can be performed in the. With regard to the classes of materials high values for tan δ are observed for absorptive materials and low values for tan δ are observed for transparent materials.
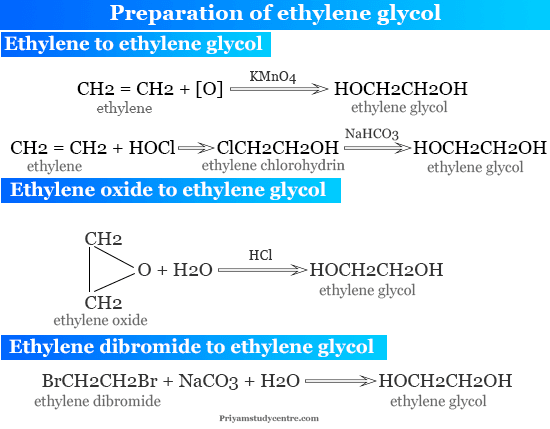
Ethylene Glycol Properties Formula Structure And Production

What Is Ethylene Glycol C2h6o2 Formula Structure Properties Uses

0 Comments